Juniper Publishers| The Anatomical Architecture of the Junction between the Great Cerebral Vein and the Straight Sinus in Correlation to the Physiological and Biochemical Impact on Multiple Sclerosis Subtypes and Disease Progression- A Review Article
Journal of Surgery- JuniperPublishers
Abstract
Introduction: Multiple Sclerosis (MS) is a debilitating autoimmune disorder, characterized by damage to the white matter of the central nervous system. Studies suggest a relationship between impaired cerebral venous blood flow and progressive destruction of white matter in the CNS. Altered cerebral venous architecture is a possible mechanism that may contribute to a compromised venous blood flow pattern in MS patients. Natural variations at junction located between the great cerebral vein (GCV) and the straight sinus (Ss) located within the cerebral venous drainage system can potentially exacerbate MS progression. Recent data indicates that abnormal cerebral venous hemodynamics contributes to the pathophysiology observed in MS through an unknown mechanism that increases hydraulic resistance.
Objective: This review aims to investigate if anatomical variations at the GSV and Ss junction elicit a profound effect on the cerebral venous system, thereby contributing to physiologic changes consistent with progressive neurodegeneration seen in MS.
Conclusion: This paper proposes a link between abnormal anatomical deep cerebral venous structures and more progressive subtypes of MS. We postulate that anatomical variations at the junction of GSV and Ss impair hemodynamics in cerebral vasculature, thus increasing immunological onslaught of the myelin sheath in advanced MS. Components of the deep cerebral vasculature system may be implicated with a more advanced disease course and increased symptomatology. These findings are of relevance when considering the progression and management of treatment of MS patients.
Keywords: Multiple Sclerosis; Great Cerebral Vein; Straight Sinus; Anatomical Variations
Abbreviations: GSV: Great Cerebral Vein; Ss: Straight Sinus; MS: Multiple Sclerosis: RRMS: Relapse Remitting Multiple Sclerosis; SPMS: Secondary Progressive Multiple Sclerosis; DCV: Deep Cerebral Venous
Introduction
Multiple sclerosis (MS) is a debilitating neurological disease of the central nervous system (CNS). Multiple sclerosis presents in individuals with a genetic predisposition, set off on a course of disease development by an environmental trigger leading to immunological destruction of myelin [1]. The insulating myelin substance wraps around the axons of neurons and is what categorizes this as white matter in the CNS. The exact cause of this immune-mediated attack on the white matter is still unknown. However, the extent of the damage caused to the myelin sheath results in varying symptomatology and severity of disease [2]. Several different subtypes of multiple sclerosis are diagnosed based upon specific criteria, which indicate the severity and course of the disease [3]. In order for a diagnosis of MS to be confirmed, there must be evidence of insult to the central nervous system in at least two separate areas, imaged with MRI, with the criterion that these attacks happened at least one month apart from each other and other differential diagnoses are eliminated [1].
Approximately 85% of all patients meeting diagnostic criteria of MS are initially diagnosed with Relapse-Remitting Multiple Sclerosis (RRMS) [3]. In RRMS, individuals suffer acute attacks demonstrating novel or exacerbating neurologic symptoms. These symptoms vary from person to person, but an individual suffering an attack may experience things such as vision troubles, bowel and bladder dysfunction, pain or weakness of the extremities, dizziness, problems concerning balance and coordination, emotional liability, problems with cognition, and fatigue. In RRMS, a patient’s attacks will be followed by a time period of either complete or partial return to the patient’s baseline with incomplete resolution of neurologic deficit [1]. Individuals with RRMS are at high risk of developing a more debilitating form of MS known as Secondary Progressive Multiple Sclerosis (SPMS). SPMS is characterized by a steady progression of disability over time, in which an individual’s neurological condition deteriorates. A person with SPMS may or may not have periods of slight recovery over time. Regardless of partial recovery these individuals are in a more advanced disease progression than those with RRMS [1].
Although most individuals meeting the diagnostic criteria of MS are initially diagnosed with RRMS, the remaining 15% of individuals are diagnosed with Primary Progressive Multiple Sclerosis (PPMS). Unlike RRMS, these individuals have an advanced and rapidly progressing form of neurologic dysfunction from the onset of disease in a similar fashion to that of SPMS. PPMS patients do not have recovery periods after attacks early in their diagnosis. However, it is possible to have later recordings of brief relief from the progressing disability [1]. These varying disease progressions and the aggressiveness of MS may be influenced by other factors such as the hemodynamic system of the individual [4]. Studies link cerebral venous vasculature abnormalities to MS and indicate the portion of the cerebral venous vasculature system that is affected more frequently amongst the more advanced forms of MS [2].
To explore the relationship between cerebral vasculature and MS, this paper focuses on the fundamental anatomical structures that might impact altered hemodynamics.The architecture of the cerebral vascular system can be split into two categories: the superficial and the deep venous drainage systems. Anatomically, the superficial cerebral venous system contains the dural venous sinuses found at the surface of the cerebellum and cerebrum [5]. The dural sinuses of the superficial cerebral venous system are connected at a single point by the joining of the superior sagittal sinus superiorly, the occipital sinus inferiorly, and the transverse sinuses laterally. This conjoining point is found in the posterior portion of the skull, deep to the occipital protuberance and is called the confluence of sinuses or torcula [6].
The confluence of sinuses is the point at which the superficial cerebral venous system connects to the Ss, and it primarily drains the deep venous system [5]. The GCV is anterior to the Ss and posterior to the midbrain and receives venous blood from the veins deep within the brain. As the composition of the deep cerebral venous system are venous structures that lie deep inside the structures of the brain, this poses a challenge to observe intact structures upon cadaveric dissection [7]. However, this central junction is a potential point of altered vascular flow that might contribute to compromised blood circulation to the deep venous system.
The difficulty of analyzing these deep venous structures intact in a cadaveric dissection places extreme importance on the procedure used. As seen in previous studies, the dissection method should remove all unintentional factors that may lead to any error in analyzing these structures, in order to maintain the deep venous vasculature stable within the brain structures. Dagain et al. [8] utilized a bi-parietal occipital craniotomy, with the dura mater incised along the lateral edges of the superior sagittal sinus and the superior edges of the transverse sinuses. This method allows the examiner to visualize these deep venous structures intact. By using this dissection technique and injecting latex, they were able to maintain the integrity of the deep structures and identify three unique variations of the junction between the Ss and GCV [8,9].
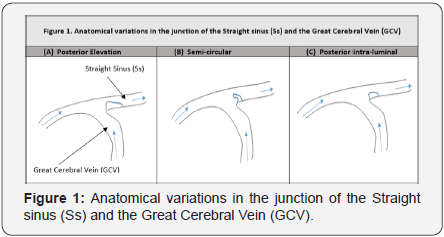
Details of the three previously described variations are illustrated in Figure 1: variation 1 is associated with an elevation in the posterior aspect of the junction (Figure 1A); variation 2 shows an outgrowth from the floor of the Ss with a semicircular form (Figure 1 B) and variation 3 presents as a posterior intraluminal nodule located in the floor of the junction (Figure 1 C). One particular study illustrated that anatomical variations within the cerebral venous system in general may be the result of influences within early stages of development [7]. Alternatively, other researchers proposed that physical and physiologic factors may be responsible for these observable variations including the diameter of the vessels, blood pressure, age, gender, and ethnicity [10]. Altered anatomical variations may result from the impact of circulating blood volumes and velocities throughout the cerebral venous system through the process known as remodeling [11]. Variations within the cerebral vascular system, including the junction between the Ss and GCV, can alter the velocities and pressures experienced within that system [12].
Variations within the architecture of an individual’s cerebral vasculature were associated with observable changes in venous blood flow in relation to those variations, visualized with transcranial color-coded duplex sonography [13]. Histological and imaging evidence revealed that anomalous venous structures were found primarily in MS patients, as compared to patients plagued with other neurological disorders [14]. Further, a connection between abnormal cerebral vascular blood flow and MS was established when cerebral blood flow in MS and controls patients, utilizing transcranial and extracranial echocolor Doppler [2]. A venous hemodynamic insufficiency severity score (VHISS) was determined by measuring the blood flow in various locations. In patients suffering from MS, the average VHISS score was 8.9 ± 2.8, in comparison to the average VHISS score in the healthy controls as 0 ± 0 (p<0.001).
Out of five distinct criteria to determine the VHISS score, patients with MS had an average of 3.8 ± 0.23 out of 5 of the criteria being met, as compared to healthy controls had an average of 0.12 ± 0.35 out of 5 of the criteria (p<0.001). One particular area of interest was measurements made from the intracranial veins, which showed abnormal blood flow in 88% of patients with MS and in 0% of healthy controls. The result from this study established a strong link between cerebral venous abnormalities, specifically the intracranial venous system, and MS [2]. A study conducted by Beggs [4] concluded that abnormal cerebral venous hemodynamics contributes to some of the pathophysiology observed in MS and that some unknown mechanism is responsible for an increase in hydraulic resistance. In patients with MS, impaired cerebral venous blood flow could consequently lead to further observable changes in the composition of white matter [4]. Subsequently, this strengthens many hypotheses which relate various factors observed in MS are secondary to abnormal venous flow in the cerebral venous vascular system.
Discussion
For almost 100 years, the importance of the GCV on cerebral hemodynamics has been implicated in neurodegenerative diseases. In fundamental study, the brains of human cadavers were injected with a hot carmine-gelatine solution under heavy pressure directly into the GCV [15]. The results showed that the injected solution leaked out into areas surrounding the lateral ventricle. Further studies examined the distribution, shape, and region-in which these extravasations occupy in Schlesinger’s work from 1939-mirror the same distribution, shape, and location of plaques seen in more progressive forms of MS [4]. These findings implicate the role that variants in the cerebral venous architecture at the junction of the Ss and the GCV may play on the progression of more advance forms of MS. Previous research analyzed abnormal venous patterns among subtypes of MS and reported remarkable differences in the presence of abnormal blood flow in the deep cerebral veins (DCV) when comparing healthy controls to all MS subtypes [1,16-18].
An evaluation of a meta-analysis combining the collective results of these studies revealed that patients with MS were more likely to have abnormal venous blood flow in the deep cerebral venous (DCV) structures. Among all 457 subjects diagnosed with MS across these studies, it was discovered that 31.51% had abnormal blood flow in the DCVs, while only 1.94% of the 310 healthy controls tested positive for abnormal blood flow in the DCVs (Figure 2). This illustrates a stark contrast between the presence of neurodegenerative disease and its connection with abnormal blood flow in the deep cerebral vasculature. Even more revealing was the prevalence of DCV abnormalities in the more progressive forms of MS. Convincingly, 43.24% of 110 PPMS and 48.18% of 37 SPMS patients exhibited abnormal blood flow in the DCVs (Figure 2). Evaluation of our collective meta-analysis of these studies revealed a higher prevalence of abnormal venous blood flow in the cerebral vascular system in the more progressive forms of MS. These data suggest that altered hemodynamics, potentially associated with variations in deep cerebral venous structures may contribute to more advanced forms of MS.

Encompassing multiple studies, data was collected and analyzed from 310 Healthy Controls, and 441 study subjects with varying subtypes of Multiple Sclerosis (MS). Among these subtypes of MS there were 310 patients with Relapse Remitting MS (RRMS), 110 with Secondary Progressive MS (SPMS), and 37 with Primary Progressive MS (PPMS). These subjects were tested utilizing transcranial echocolor Doppler for the presence of abnormal blood flow in the Deep Cerebral Venous (DCV) system as shown by the percentages (%) above.
Further evidence implicating abnormal cerebral venous blood flow in MS is evident in a study that examined 313 patients with a known diagnosis of MS in conjunction with a previous diagnosis with an aberrant venous blood flow pattern [19]. Of the patients with MS, 95.53% showed abnormal blood flow at the level of the deep cerebral venous system. Further analysis shows that among the subtypes of MS noted, SPMS and PPMS had the highest prevalence of abnormal blood flow in deep cerebral venous system at 96.7% and 98%, respectively. As expected, these individuals also possessed a higher Expanded Disability Status Scale (EDSS) score, coinciding with a more advanced neurologic deterioration in the individual - suggesting a link between abnormal deep cerebral venous blood flow and a more debilitating form of MS quantified by neurologic examination [19]. Additionally, the EDSS overall showed an upward trend associated with more advanced forms [1,16-18].
With the collective findings that cerebral vascular flow is compromised in MS patients, the mechanism that triggersvascular damage is poorly understood. Many histological studies have confirmed that MS plaques are venocentric [20,21]. Histological evidence has furthermore visualized iron deposits present in the outer venous vasculature in MS patients [22,23]. Copious iron poses tremendous risk to the surrounding brain and spinal cord tissue due to the well-known Fenton reaction. Free and stored iron has the electric potential to generate reactive oxygen species and free radicals in this reaction. These reactive oxygen species create oxidative stress, which can damage neurons and the surrounding myelin sheath [24].
Iron deposit stores are also involved in another pathological mechanism associated with MS. These iron deposits are essentially packets of heme which originate from erythrocytes, which undergo extravasation - especially during recent MS relapses - to help form the encircling MS plaques [22,25]. Diapedesis, leukocyte extravasation, is a mechanism of the innate immune system, in which non-specific leukocytes move out of the systemic circulatory blood vessel into the surrounding tissue towards a chemokine stimulus. Histological staining of MS plaques in one study illustrated that iron deposits act as a strong chemoattractant stimulus for macrophages, leading to ironladen phagocytes within these MS plaques [26]. Nevertheless, numerous red blood cells, macrophages, and lymphocytes are commonly noted in the extravascular CNS tissue in MS patients. MRI imaging reveals that the blood brain barrier is more permeable, especially during attacks, thus permitting immune cells access to the vulnerable myelin [27,28].
The iron-induced oxidative stress compromises the integrity of the blood brain barrier [28] and creates a positive feedback mechanism by replenishing its erythrocyte stores. Additionally, heme concentrates a mass of phagocytes, including activated macrophages, which consequently damage the surrounding parenchyma. The main cytokine in neurodegeneration is IFN-Υ produced by T helper cells [29]. Fortunately, under normal physiological conditions, the T lymphocytes self-limit the compounding effect of their secreted IFN-Υ through a negativefeedback loop. IFN-Υ induces apoptosis of its own and nearby creators. Iron has been noted to disrupt the IFN-gamma/STAT- 1 signaling [30]. Thus, iron deposits reinforce the initial trigger in MS by prolonging the cellular lifecycle of the autoreactive T lymphocytes and consequently increasing concentrations of IFN-gamma; and leading to more neuronal damage. All of the effects on neuronal tissue in MS by iron deposits, autoreactive T cells and interferon-Υ induced iron-laden macrophages are greatly potentiated by the extravasation of their effectors from the systemic circulation.
Anomalous venous infrastructure has been found to greatly increase extravasation. MS is significantly associated with chronic cerebrospinal insufficiency, which includes stenosis of numerous major venous vessels and reversal of flow in the deep cerebral veins [16]. Normal, stable laminar flow of blood and the shear stress it creates on the vascular wall stimulates theendothelium to create and release factors that inhibit coagulation and leukocyte transmigration [31]. Conversely, when the blood flow becomes turbulent or the direction of flow reverses, the endothelium responds as if reacting to inflammation. Specifically, it up-regulates the expression of leukocyte surface adhesion molecules on the endothelium of the blood-brain barrier [31]. Consequently, this leads to extravasation of leukocytes and plasma contents (including demyelinating autoantibodies and complement), giving rise to the edematous destruction of the white matter commonly seen in MS patients. Therefore, the reversal or oscillatory flow in the cerebral venous system is a pro-inflammatory stimulus [14].
Of particular interest is the oscillatory flow in the deep cerebral veins, where the junction of the GCV and Ss is found. Reflux in the deep cerebral veins in the GCV and Ss junction was found in 50% of MS patients and 0% of all other neurodegenerative, neuro immune, and neurodegenerative vascular diseases (p<0.0001) [16]. Furthermore, studies have found that MS develops particularly along the CNS parenchyma following the reflux pathways in venous vasculature [32,33].
Conclusion
A growing body of research data is supportive of the direct association between cerebral hemodynamic abnormalities and neurological degenerative diseases with evidence indicating that the cerebral venous system is one of the most significant variables in standard brain function [34]. In this review, we examined existing research linking neurological disease with cerebral vascular disorders and variations in the anatomical architecture of the cerebral venous system.
Anatomical variations in the cerebral venous structure may impact blood flow and pose adverse effects on individuals with preexisting neurological degenerative diseases [4]. These findings strongly support that anomalous variants of the junction between the GCV and Ss may greatly increase the risk for more progressive forms of MS or exacerbate the current MS symptoms. If one of the junction variants slows the flow of blood, many contents in the blood, including iron, may initiate the inflammatory chain response and ensuing damage to the white matter. More specifically, if a variant creates a stenosis effect and a subsequent reversal of flow, it acts as a pro-inflammatory stimulus. Thus, the endothelium upregulates leukocyte adhesion molecules, which facilitate the rolling, adhesion, and transmigration of macrophages and T lymphocytes into the CNS parenchyma of that particular area, are yielding its destruction. As a result, this is considered a critical vascular element in the progression of MS [35].
We postulate a correlative relationship between altered cerebral blood flow due to variations in the anatomical architecture at the junction of the straight sinus (Ss) and great cerebral vein (GCV), increased immunological insult to the cerebral white matter and severity of symptoms in MS patient symptoms. However, further data analysis should be done to evaluate the relationship between the EDSS of MS patients with anomalous cerebral venous blood flow and MS patients with normal cerebral venous blood flow to better understand the effects that aberrant cerebral venous blood flow may have on EDSS. In future studies, accommodations should be made to identify the anatomical variation present at the location of the GCV and Ss, the relation of the structure to MS subtype, and its impact on progression of disease.

Comments
Post a Comment