Juniper Publishers| A Review of Indication and Complications of Extracorporeal Membrane Oxygenation
Journal of Surgery- JuniperPublishers
Abstract
Mechanical circulatory support may be the last life-saving resort in certain circumstances such as life-threatening pulmonary failure, cardiac failure, or both. ECMO has been shown to provide adequate cardiopulmonary support and can be initiated rapidly in an emergent setting by either percutaneous or surgical implantation. It allows bridging of patients with cardio-pulmonary collapse to recovery or long-term mechanical support. Veno-arterial ECMO (VA-ECMO) is used as bridge-to-decision and/or bridge-to-recovery in patients with cardiogenic shock. Long-term mechanical circulatory support devices such as left ventricular assist devices (LVADs) are widely available and play a central role in bridge-to-transplantation in those eligible for heart transplantation (HTX) and as destination therapy (DT) in those not eligible for HTX. LVAD-implantation or HTX in patients with acute cardiogenic shock is associated with dismal outcomes; this illustrates the importance and necessity for short-term support like ECMO. This manuscript provides an overview of indications, outcome, and complications of ECMO.
Keywords: Acute cardiogenic shock; Mechanical circulatory assist device; Extracorporeal membrane oxygenation; Bridging to transplantation; Left ventricular assist device
Abbreviations: VA-ECMO: Veno-Arterial ECMO; LVADs: Left Ventricular Assist Devices; HTX: Heart Transplantation; DT: Destination Therapy; CPB: Cardiopulmonary Bypass; VV: Venovenous; VA: Venoarterial; PCI: Percutaneous Coronary Intervention; VAD: Ventricular Assist Device; IABP: Intra-Aortic Balloon Pump; AFM: Acute Fulminant Myocarditis; AKI: Acute Kidney Injury; CRRT: Continuous Renal Replacement Therapy; ELSO: International Extracorporeal Life Support Organization; APACHE: Acute Physiology and Chronic Health Evaluation
Historic Background
It is controversial to refer to ECMO as a cardiopulmonary bypass (CPB); however, ECMO is based on the same principle -- in other words, ECMO is a variation of CPB and may be viewed as prolonged cardiopulmonary bypass, allowing for a prolonged cardiopulmonary support [1,2]. The history of ECMO is date back to invention of CPB. Gibbon attempted to create a heart-lung machine and designed an oxygenator, where the anticoagulated blood was exposed directly to oxygen (bubble oxygenators). However, this approach caused severe hemolysis, thrombocytopenia, hemorrhage and multiorgan failure [3]. In 1956, Clowes et al. [4] introduced the membrane oxygenator that separated blood from oxygen by a membrane, securing efficient and safe blood oxygenation and fewer complications compared to film or bubble oxygenators. In 1983, Larm et al. [5] introduced a new ECMO system in which heparin molecules are covalently bonded to the synthetic surfaces of the ECMO circuit. The heparin coating of the ECMO circuit comes into contact with the blood, reducing complications significantly [6]. In a prospective randomized controlled study, Knoch et al. [7] reported that the use of heparin-coated circuits and oxygenators reduces blood loss and the need for blood transfusion. Introduction of heparin-coated ECMO circuit revitalized this technology.
Indication
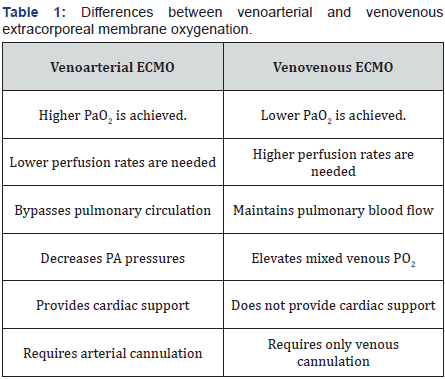
Fundamentally, ECMO is used as a bridge to an eventual recovery in patients with cardiogenic shock [8-10]. The indication for ECMO has been divided into three major categories: cardiogenic, respiratory, or cardiopulmonary failure. There are two primary forms of ECMO, based on the indication and the type of access: venovenous (VV) and venoarterial (VA). Table 1 demonstrates some of the characteristics of VV- and VA-ECMOs [1]. ECMO management (i.e., how ECMO should be utilized once initiated) is based on consensus guidelines and institutional experience; however, there are not established guidelines regarding the indication and timing for the initiation of ECMO support and the approach should be individualized to each patient’s condition [2,11]. ECMO can also be used as temporary hemodynamic support in order to perform an invasive cardiac treatment (percutaneous coronary intervention (PCI)), or to assess the eligibility of the patient for LVAD placement [1,2].
A. Cardiogenic shock
ECMO is indicated for the management of refractory cardiac or cardiopulmonary failure. While it is used in a variety of clinical scenarios, the outcome often depends on the primary indication [15,19,20]. Postcardiotomy shock, the main indication for ECMO in adult populations (occurs in 0.5% to 1.5% of cases), is defined as the inability to wean the patient from extracorporeal circulation using inotropic and vasoactive drugs as well as an intra-aortic balloon pump (IABP) [20-22]. ECMO may allow the affected organ to recover (bridge to recovery) or provide enough time for the decision (bridge to decision) and prepare the patient for a long-lasting organ substitution either by a ventricular assist device (bridge to bridge, or destination LVAD) or transplantation [23]. ECMO can be used to bridge patients with end-stage heart failure to heart transplant [12,24]. Earlier initiation of ECMO may prevent complications and improve outcomes [25]. It provides temporary assisted circulation, (partial) off-loading of LV, and respiratory support to prevent multisystem organ failure and death [10,21,26].
B. Acute fulminant myocarditis
Acute fulminant myocarditis (AFM) carries high mortality and those affected are candidates for short-term mechanical support. Though highly morbid, most patients with AFM recover and are weaned from ECMO [27]. In a retrospective review of Extracorporeal Life Support Organization Registry database (n=19,348 patients), 260 ECMO patients (1.3%) had AFM. Survival to discharge from the hospital was 61%. The female gender, arrhythmia while on ECMO, and acute renal failure requiring dialysis were predictors of worse outcomes in patients with AFM who received ECMO [28]. In a different series of 75 patients with AFM, the survival to discharge was 64% (n = 48). Pre-ECMO resuscitation did not have a negative impact on survival [27]. In a larger series of patients with acute cardiogenic shock, 11 patients had fulminant myocarditis. Eight patients recovered and one was successfully transplanted. All three deaths were due to neurologic complications [29].
Furthermore, using ECMO during pregnancy was discouraged in the past, with the assumption of increased risk of bleeding as well as increased fetal and maternal mortality. However, a recent metaanaylsis demonstrated the safety of ECMO for severe cardiopulmonary failure during pregnancy. The overall maternal and fetal survival on ECMO has been reported to be about 80% and 70%, respectively. Meticulous anticoagulation at lower therapeutic levels may be required [30].
C. Septic shock
ECMO is a valuable therapeutic option for adults in severe septic shock with refractory cardiac and hemodynamic failure. Brechot et al. [28] reported using ECMO for hemodynamic support of patients with severe septic shock refractory to medical management. All patients had severe myocardial dysfunction (EF10%-30%). Twelve patients (86%) could be weaned off ECMO after 5.5 days of support and 10 patients (71%) recovered to be discharged from hospital and remained alive at a mean follow up of 13 months [28]. However, Huang et al. [31] reported a series of 52 adult patients in refractory septic shock, who received VA-ECMO, with only 15% (n=8) survival. The authors reported that using ECMO in adult patients with refractory septic shock is associated with unsatisfactory outcome [31]. ECMO has also been utilized to stabilize adult trauma patients in the presence of coagulopathy and/or brain injury. The benefits include rapid re-warming, acid base correction, oxygenation, and circulatory support [28].
D. ECMO after Heart Transplant, Acute Graft Failure
Acute graft failure following heart transplant is a serious complication and ECMO support might be beneficial. A retrospective review of 385 consecutive heart transplants revealed that 46 patients suffered acute graft failure requiring ECMO support. The overall success rate, defined as removal of ECMO, was 47.9%; 51.4% for early graft failures and 50% for late graft failure, while the long-term outcome remained similar. Any graft failure requiring mechanical support is associated with high mortality and unfavorable short- and long-term outcome [32].
E. TAVR
Considering the evolving field of enodvascular procedures, and the complexity of procedure performed in multimorbid ever aging patient population, ECMO may provide adequate short term cardiopulmonary support during TAVR on emergency or prophylactic bases. High Euro-score might be an indication for using prophylactic VA-ECMO support during complex endovascular procedures. Life-threatening complications during TAVR can be managed using emergency VA-ECMO but mortality remains high [33].
F. Hypothermia
ECMO has been used to resuscitate patients with severe accidental hypothermia with or without cardiac arrest (n=26). Sawamoto et al. [34] reported a survival rate of 38.5% in a series of 26 patients. While neurological outcome was generally acceptable at discharge; a cardiac rhythm other than asystole, nonasphyxial hypothermia, higher pH, and lower serum lactate were associated with more favorable neurological outcome [34]. Considering these patients’ condition prior to initiation of ECMO, the neurologic injury may not be an immediate complication of ECMO, rather a feature of their condition on presentation.
G. Technical aspects
The cannulation can be done either peripherally or centrally. After gas exchange in vitro, the blood is returned either peripherally or centrally into the patient’s venous or arterial system, depending on type of ECMO [35]. Components utilized for conduction of ECMO include: a pump, an oxygenator, and a circuit. The oxygenation of blood occurs via a membrane, which is a cylindrical rotor comprised of a strong textile support coated by a plastic microporous film [36]. Currently, the most efficient systems utilize a small centrifugal pump and a low-resistance polymethylpentene-oxygenator. Models such as the RotaFlow (Maquet, Jostra Medizintechnik AG, Hirrlingen, Germany) and CentriMag (Levitronix LCC, Waltham, MA, USA) are used for this propose [37]. ECMO flow depends on the available volume in the heart chambers, the speed of the pump, and the vascular resistance. Attention should be paid to avoid hypovolemia, cannula malposition, pneumothorax, and pericardial tamponade.
H. Central vs peripheral cannulation
ECMO can be placed either centrally (Figure 1) through a sternotomy or peripherally, percutaneous (Figure 2), frequently using the femoral artery and vein [38]. Central ECMO cannulationis achieved with direct cannulation of the aorta and provides antegrade flow to the arch vessels, coronaries, and the rest of the body. In contrast, the retrograde aortic flow by peripheral ECMO leads to mixing of the blood in the arch or descending aorta and may not adequately supply the arch vessels with oxygenated blood. In certain patients with cardiac or respiratory failure who have recently undergone cardiac surgery, transthoracic cannulation of the right atrial appendage and the ascending aorta is performed. A disadvantage of central cannulation is that the chest must be left open which is associated with increased risk of infection and bleeding. Newer cannulae are designed to be tunneled percuatneously through the subcostal margin and abdominal wall, allowing the chest to be closed. Transthoracic cannulation may allow better left heart decompression and oxygenation of vital organs.
On the other hand, percutaneous cannulation is less invasive and can be performed at the bed side with the guidance of transthoracic echocardiogram [39]. The major disadvantages of the transthoracic approach are vascular injury during cannulation and ischemia of ipsilateral lower extremity distal to the cannulation site. ECMO through peripheral vessels is associated with early [40] and late vascular complications at the femoral access site [41]. However, some authors advocate peripheral cannulation in selected patients. Using femoral vessels is an established access route for peripherally-inserted ECMO. However, in some patients, alternative cannulation sites should be considered [42]. However, in a retrospective series of 50 ECMO cases, the cannulation was performed either by central or peripheral cannulation. The authors did not report any difference in the incidence of ischemia or compartment syndrome in the lower extremities, while central cannulation was associated with a higher risk of bleeding, need for transfusion, and greater utilization of resources. There was no difference in 30-day mortality [38]. Difficulty during ECMO placement or removal and a history of peripheral vascular disease are predictors of long-term vascular complications [26,41]. Symmetrical peripheral gangrene is an unusual complication of ECMO that may arise in the setting of DIC, sepsis, or other hemostatic and/ or hemodynamic imbalance [40]. Minor vascular complications after ECMO support are not associated with higher mortality rates [43].
I. ECMO via axillary artery
ECMO via axillary cannulation, though still considered peripheral, allows for antegrade perfusion and supplies the aortic arch vessels with adequately oxygenated blood. Axillary artery cannulation is commonly performed through a Dacron graft sutured in an end-to-side fashion to the axillary artery. Direct cannulation of the axillary artery is a reliable option with an acceptable complication rate [44-47]. Exposure of the artery is achieved via the deltoid-pectoral approach. Advantages of axillary artery cannulation are the nearly central cannulation with antegrade perfusion and excellent upper body oxygenation. It also allows for chest closure after postcardiotomy shock or avoids the sterntomy in the first place [45]. Axillary cannulation is a viable option, especially in patients with significant peripheral vascular disease, [45,48] and may avoid many complications inherent to transfemoral ECMO. However, hematoma formation has been reported which may cause injury to the nearby brachial plexus [48].
In a series of 308 adult ECMO patients, axillary artery cannulation was performed in 81 patients (26.3%), 166 patients (53.9%) received femoral arterial cannulation, and 61 (19.8%) underwent ascending aortic cannulation. The most common complication following axillary cannulation was hyperperfusion syndrome of the ipsilateral upper extremity (up to 25%), followed by bleeding from the arterial outflow graft. Lower extremity ischemia and fasciotomy were the most frequent complications after femoral arterial cannulation [49]. Wada et al. [50] compared the efficacy of transfemoral with transaxillary ECMO in a canine model. Percutaneous cardiopulmonary support was initiated via the left femoral artery and then switched to the right axillary artery. Cerebral tissue oxygen saturation was 54.2±3.4% with femoral artery cannulation verses 82.3±4.6% during axillary artery cannulation. LV dP/dt max as a sign of myocardial contractility increased significantly after switching to the axillary perfusion [50].
Comparing central (n=65; 53.7%) vs peripheral (n=55; 46.2%) cannulation for ECMO, Loforte et al. [37] reported overall survival of 64.7% (n=77), weaning from mechanical support (n=51; 42.8%) and bridge to heart transplantation (n=26; 21.8%). Regardless of cannulation site, the overall mortality was 35% (n=42). Serum lactate levels, creatine kinase-MB relative index at 72h after ECMO initiation and number of packed red blood cells (PRBCs) transfused on ECMO were predictors of mortality. The central ECMO cannulation group had a higher rate of bleeding events compared with the peripheral cannulation group [37]. Saeed et al. [51] compared peripheral access (n=25) with the central approach (n=12) in 37 patients. While, 11(44%) of the pECMO patients required exploration for bleeding compared to 100% of patients with cECMO. The same study reported a 30-day mortality in patients with pECMO and cECMO of 60% versus 67%, respectively [51].
J. Complications of ECMO
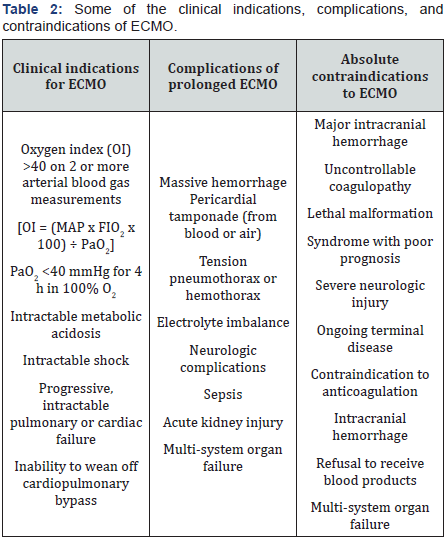
ECMO complications include those associated with cannulation (pneumothorax, vascular disruptions, bleeding, infection, emboli), systemic anticoagulation (GI bleeding, intracranial bleeding, etc), and exsanguination resulting from circuit disruptions. The majority of complications fall into one of three major categories: bleeding, sepsis with multisystem organ failure, or neurologic sequelae. Bleeding and hemolysis, which are out of proportion to the severity of coagulopathy, may occur while on ECMO support; [25,26,52-54] cardiac tamponade and acute renal insufficiency may also occur [21]. Some of the major complications and contraindications are shown in (Table 2) [9,18,22,27,55-63].
In a meta analysis which included 12 studies (n=1763 patients), the most common complications associated with ECMO were renal failure/dialysis (occurring in 52%), bacterial pneumonia (33%), bleeding (33%), oxygenator dysfunction requiring replacement (29%), and sepsis (26%). Pneumonia, sepsis, arrhythmia, and multisystem organ failure comprise additional complications of prolonged ECMO support [54-57]. In a series of 117 postcardiotomy ECMO patients, the most common complications were re- exploration for bleeding (n = 24), alimentary tract hemorrhage (n = 14), renal failure requiring renal replacement therapy (n = 29), infection (n = 32), limb ischemia (n = 5), oxygenators malfunction (n = 29), and hemolysis (n = 7). Overall, 87 patients (74.4%) were successfully weaned from ECMO and 69 patients survived to discharge [25]. In another series of 129 patients undergoing VA-ECMO 59 patients (38%) were weaned, 7 (5.4%) were bridged to a LVAD, and 6 (5.2%) were listed for heart transplantation [64]. Overall mortality was reported as 54%, with 45% of events during ECMO support and 13% after weaning.
Finally, In a metaanalysis by Cheng et al. [65] including 1,866 patients, major complications were: lower extremity ischemia (16.9%), fasciotomy or compartment syndrome (10.3%), lower extremity amputation (4.7%), stroke (5.9%), neurologic complications (13.3%), acute kidney injury (55.6%), need for hemodialysis (46.0%), major or significant bleeding 40.8%, rethoracotomy for bleeding or tamponade in postcardiotomy patients (41.9%), liver dysfunction, and infection (30.4%). Survival to hospital discharge ranged from 20.8% to 65.4% [65]. Device-related complications include tubing rupture, pump malfunction, oxygenator failure, heat exchanger malfunction, and cannula-related problems [66]. Lewandowski et al. [67] reported a total of 27 device related complications during 27,137 hours of ECMO support. The documented complications included pump malfunction (n=6), tubing rupture (n=6), and cannula placement or removal problems (n=5) [67].
a) Bleeding
Continuous activation of fibrinolytic systems by the circuit and consumption and dilution of coagulation factors may occur. Platelets may adhere to the circuit surface and become activated, resulting in platelet aggregation, clumping, and subsequent thrombocytopenia [68]. A plasma free hemoglobin > 10 mg% may indicate hemolysis [69]. Bleeding seems partly related to intravenous heparinization, [25,26,53,54,70,71] and can be managed in any standard situation by decreasing or stopping heparin and infusing platelets and blood products. However, increased red blood cell transfusion is associated with adverse outcomes in ECMO patients [70]. Smith et al. [70] reported in 484 ECMO runs with a median duration of ECMO support of 4.6 days that patients with post cardiotomy cardiogenic shock required increased RBC transfusion compared to other patients with cardiogenic shock. A higher RBC transfusion rate carried higher in-hospital mortality. A lower baseline hematocrit and increasing duration of ECMO support were risk factors for increased RBC transfusion [71]. Regular monitoring of coagulation profile, platelet count, hemoglobin, and creatinine are the routine, while an early replacement of clotting factors and electrolytes may prevent complications [69]. In addition to bleeding at the surgical site, other bleeding related complications include intracerebral bleeds [9,18,22,27,55-63].
b) AKI
Acute kidney injury (AKI) is a major complication and is associated with high mortality in adult ECMO patients. Oliguria followed by acute tubular necrosis occurs with AKI, which may require hemofiltration and dialysis [56-58]. The impact of fluid status, while on ECMO, was evaluated in 115 patients with acute cardiogenic shock and 57 patients with refractory respiratory failure. Fifty-seven per cent of patients had acute kidney injury (AKI) after ECMO initiation, and 60% (n = 103) of patients received hemodialysis/continuous renal replacement therapy (CRRT) during ECMO course. Overall 90-day mortality was 24%. Acute Physiology and Chronic Health Evaluation (APACHE) III, CRRT during the first 3 days, major bleeding, and positive FB was independent predictors of 90-day mortality [72]. ECMO may alter serum concentration of drugs due to increased volume of distribution, which makes dose adjustments necessary [56,57].
K. Neurologic Complications
Neurologic adverse events range from gross motor delay to spastic quadriparesis and seizures. Intracranial bleeds, and hemorrhagic as well as embolic stroke, remain the main culprits for neurologic adverse events. Intracranial hematoma, while on ECMO, is a serious complication. Neurologic adverse events occur in approximately 7-15% patients [25,73,74]; while, systemic heparinization, thrombocytopenia, coagulopathies, and systolic hypertension are the major risk factors [73]. Although the risk is substantial, surgical evacuation of hemorrhage might be indicated in some patients [73]. Management of intraparenchymal hemorrhage while on ECMO has limited success and carries high rates of re-bleeding and in-hospital mortality (75%). Krenzlin et al. [75] reported such complications in 12 patients; 11 LVAD, and one ECMO. Surgical hematoma evacuation was performed in 11 patients; one patient received decompressive hemicraniectomy [75]. Reversing the anticoagulation may be lifesaving in those patients. Acidosis, renal failure, and cardiopulmonary resuscitation increase the risk of neurologic complications [76].
The survivors of prolonged ECMO may suffer from longterm neurologic sequelae, regardless of manifested neurologic complications during the time of ECMO support as noted [77,78]. Indeed, The intelligence quotient (IQ) scores of patients who received ECMO have been reported to be lower [74]. Moreover, ECMO is associated with an increased prevalence of long-term psychiatric disorders and distress [76,79]. While neurologic complications occur with prolonged ECMO support, in some patients, ECMO can prevent cerebral hypoperfusion and actually improve neurologic outcome after cardiac arrest.
L. Vascular complications
Peripheral vascular complications occur in less than 20% of patients and are more common with peripheral cannulation [80]. Among those patients who develop vascular complications, the most common indication for ECMO is cardiogenic shock [80]. In a series of 100 VA-ECMO patients, the majority of ischemic episodes were resolved or prevented with the insertion of a distal perfusion catheter [81]. An adequate distal limb perfusion via a 6-8 Fr cannula, placed in distal femoral artery, may reduce the risk. In a series of 83 ECMO patients; 45 received peripheral VA-ECMO. Distal limb perfusion was achieved with an introducer sheath (6-8 Fr) in 13 cases and with a distal-perfusion cannula (10-12 Fr) in 32 cases. Nine (20%) patients developed signs of ischemia; five (11.2%) were treated conservatively, while four (8.8%) required surgical intervention. The incidences of limb ischemia and limb ischemia requiring surgical intervention were significantly lower using the introducer sheath compared with the cannula [82]. In 101 patients receiving ECMO, vascular complications were observed more frequently in male patients (78%), in those receiving prolonged ECMO support, and in patients with chronic CHF (72%). Overall mortality was 42% (n=42) [80].
M. Infection on ECMO
Prolonged ECMO support is associated with higher infection rate [83]. In a series of 139 patients undergoing ECMO, 36 patients had a total 30 infectious episodes per 1,000 days of ECMO. Enterobacteriaceae and Candida were the most frequent pathogens. Infection did not significantly increase the risk of mortality, but the length of stay in the ICU and in hospital were prolonged following infection [83].
N. Risk Factors for Adverse Outcome
Wang et al. [84] reported 59% of patients (n=87) were successfully weaned from VA-ECMO and 49% were discharged. Older age (>65 years), postoperative hemodialysis, a peak lactate level (> 12 mmol), LVEF <40%, and prolonged ECMO support (>60 hours) were independent predictors of in-hospital mortality. IABP placement had a favorable impact on survival [84]. Prolonged ECMO support (48 hours) and incomplete sternal closure were significant risk factors for mortality [59]. Slottosch et al. [39] reported an overall 30-d mortality rate of 70% in 77 patients who required ECMO following cardiotomy. Age at ECMO implantation, high lactate levels, prolonged ECMO support, and gastrointestinal complications were independent predictors for 30-day mortality [39].
ECMO-assisted PCI for patients with AMI complicated by profound CS was shown to improve the 30-day and 1-year survival rates [85]. The 6-h pH value at the time of ECMO was an independent risk factor of 30-day mortality. Neither CPR nor implantation under ongoing CPR results in significant differences in outcome [86]. In a series of 129 patients undergoing VAECMO, myocardial function improved significantly. Lower dose of inotropes before ECMO was a positive predictor of weaning from ECMO. Longer ECMO support, transfusion rate, and central cannulation were predictor of unfavorable outcome, even after successful weaning from ECMO; 15 (31%) patients died following weaning from ECMO. Central ECMO, persistent RV failure, need for dialysis, higher inotropic score, lower systolic pressure, or higher leukocyte count at weaning correlated with mortality [64]. In a metaanalysis (12 studies, n=1763 patients), mostly VA-ECMO, for various pathology, the 30 day mortality was 54%; 45% while on ECMO and 13% after weaning. Almost 50% of patients receiving ECMO survived to be discharged.
O. ECMO and IABP
One important pitfall of ECMO is the inappropriate offloading of the LV, leading to pulmonary stasis and inadequate myocardial recovery. Off-loading the LV is crucial for possible myocardial recovery. Barbone et al. [87] used a 7F pigtail to offload the LV. The pigtail catheter was placed in LV through the aortic valve. The authors reported that this approach provided a better off-loading of LV and prevented distension as well as lung congestion [87]. Simultaneous IABP or a left ventricular vent may improve the LV off-loading [88]. Ma et al. [88] reported the benefits of IABP in 54 ECMO patients; 31 patients received IABP followed by ECMO, and the remaining patients had ECMO placement first prompted by LV distention and minimal opening of the aortic valve. The authors reported favorable outcome combining ECMO and IABP [88]. We recommend using a simultaneous IABP to offload the LV and improve the outcome.
P. Age
Survival to hospital discharge is lower in elderly patients (>65 years) who received ECMO support compared to younger adult patients (28.7% vs. 40.0%). In one study, a total of 212 patients received ECMO for cardiac (n = 126) or respiratory (n = 86) failure. The overall survival to discharge was 33%; older age, chronic cardiac related issues, and transfusions were predictors of unfavorable outcome. In another study, age was a significant risk factor in cardiac patients [89]. However, advanced age should not be an absolute contraindication for ECMO. The International Extracorporeal Life Support Organization (ELSO) registry database was used to investigate ECMO support among 99 elderly patients (>65 years of age, median age of 70 years). For survivors, the median time spent on ECMO was 69 hours, and the median time to discharge spent off ECMO was 587 hours. Overall, survival at hospital discharge was 22.2% (22/99). The presence of acute renal failure, which preceded ECMO placement, was found to be the most significant risk factor for mortality [90].
Q. Comment
Despite substantial mortality, ECMO implantation in selected patients might be a life preserving measure that otherwise would not survive. ECMO may result in recovery in 40%-60% of the patients with cardiogenic shock; this survival rate has remained stable over the past decade [9,18,22,27,55-63]. The favorable long-term outcomes of ECMO survivors may justify itsuse; however, the decision to utilize ECMO should be made on case by case bases depending on patient’s individual risk profile [84]. Patients with chronic heart failure may have structural remodeling in the myocardium and increased interstitial fibrosis, which results in a combined systolic and diastolic dysfunction [91]. These patients may not recover on ECMO and a long-term plan should be in place before placement of ECMO in this patient population. A preoperative poor left- ventricular ejection fraction, [92] systolic blood pressure <90 mmHg, poor myocardial systolic function, and severe refractory metabolic acidosis are associated with poor outcomes [93].
Although ECMO is associated with adverse events, the patients underlying condition may contribute significantly to this adverse event profile. Therefore, the terminology “ECMO mortality” most likely reflects the combined mortality of the patient’s already poor condition as well as ECMO itself, not ECMO alone. The efficacy of ECMO would be better evaluated in a prospective randomized clinical trial; however, given the end point of such a study would be death, a study of this kind cannot be ethically justified. Furthermore, only a small number of adult patient populations receive ECMO support, which makes it difficult to study the outcome and management of ECMO [94].
Once patients survive the acute phase of ECMO implementation, they have a reasonable long-term survival, [59] depending on the etiology of their underlying disease. Survival on ECMO depends on multiple factors such as patient’s age and etiology of the disease [55,64]. In a diverse group of 45 patients with cardiogenic shock who required temporary ECMO support, 27 patients could be successfully weaned from support (60%); additionally, five were bridged to heart transplantation. The in-hospital mortality was 42% (19/45). The main cause of death remained multisystem organ failure [26]. In a series of 108 patients with postcardiotomy cardiogenic shock [55], pediatric patients had the best survival (65.7%), followed by patients who suffered primary graft failure following cardiac transplantation (42.9%). The worst survival rates were observed with postcardiotomy shock (15.2%). Older age, high body weight, increased AST/GOT levels, thrombocytopenia, and higher serum creatinine were predictors of mortality [55]. These findings have been confirmed by other authors [60]. In addition, greater lactate levels after 24 h of ECMO therapy, lactate level during ECMO support, urine output, postoperative need for dialysis, serum total bilirubin >6 mg/dL, mean arterial pressure (MAP), duration of ECMO, low serum albumin level, low oxygen pressure of the venous tube of the ECMO, and pre-ECMO EF < 30% seem to be significant predictors of hospital mortality [22,60,62,63,78,93].
In a larger series of 137 ECMO patients, 39% of patients survived to be discharged home. Male gender, a longer duration of mechanical ventilation before initiation of ECMO, and renal or hepatic failure while on ECMO were risk factors for higher mortality [95]. Zangrillo et al. [96] did a meta-analysis including 12 studies (1763 patients) and reported a 30-days mortality of 54%; of which 45% occurred while on ECMO and 13% after weaning and explantation of ECMO [96]. Acute Physiology and Chronic Health Evaluation (APACHE) IV scores have been reported to have a prognostic value in patients who required postcardiotomy ECMO [60].
Patient selection, based on preoperative parameters and underlying condition of patients, though difficult, may be a major determining factor for a favorable outcome [97]. ECMO placement in a report of 517 postcardiotomy cardiogenic shock patients showed high weaning rate of 63%, while 24.8% were discharged from the hospital. Neurologic complications occurred in 17%. Age greater than 70 years, DM, preoperative renal insufficiency, EuroSCORE greater than 20%, and a lactate level greater than 4 mmol/L were significant risk factors of mortality. Isolated coronary artery bypass grafting had a positive impact on outcome [22]. Lan et al. [61] reported in a larger series of 607 adult patients that age, stroke, the need for dialysis during ECMO, pre-ECMO infection/bactermia, hypoglycemia, and alkalosis were independent predictors of mortality [61]. Wang et al. [59] reported a series of 62 patients who required temporary postcardiotomy ECMO support: 40 patients (64.5%) were successfully weaned from ECMO and 34 patients (54.8%) were discharged from hospital. With an in-hospital mortality of 45%, the main cause of death was multisystem organ failure [78]. Early ECMO placement may improve the outcome, i.e. successful weaning as well as shorter hospital stay [98] while, prolonged use of ECMO with refractory cardiac failure, respiratory failure, or both is associated with reduced survival [60].
Summary
For some patients with acute cardiogenic shock or pulmonary failure, ECMO is the last life preserving option. It may provide partial biventricular off-loading as well as respiratory support. A recovery without the need for long-term extracorporeal circulation may be achieved in selected patients. Early implementation, control of complications, and meticulous management during ECMO and explantation of ECMO in a timely fashion can improve the patient outcome. Early placement may improve outcomes and give clinicians the necessary time for further work-up and bridge the patients to decision.

Comments
Post a Comment