Juniper Publishers| A New Clinical Classification of Hilar Cholangiocarcinoma (Klatskin Tumor)
Journal of Surgery- JuniperPublishers
Introduction
Altemeir first described hepatic hilar cholangiocarcinoma in 1957 [1]. In 1965, a series of 13 cases of hepatic hilar cholangiocarcinoma was reported by Klatskin [2]. This tumor makes up about 60% of all cholangiocarcinoma. Anatomically, this tumor situates at special site, i.e. hilar biliary bifurcation within a limited small space, close to vessels (portal vein, hepatic artery) and liver (especially caudate lobe). Biologically, it usually grows slowly and locally, with submucosal infiltration (up to 1.6 cm from gross margin of the tumor), neurovascular infiltration and lymphnode metastasis, but less often with distance metastasis. Therapeutically, its resection usually is difficult, especially to obtain R0 resection, while it does not respond well with chemo-and/or radiational therapy. Local recurrence is high (>50%), leading treatment failure and poor outcome [3].
When humans ingest the eggs of the tapeworm, embryos emerge from the eggs and penetrate the intestinal mucosa. From there the embryos are transported via the blood to various organs and get lodged in organs like liver (65 - 75% of all cases) and lungs (25 - 30% of all cases). In children the most common organ being lungs followed by liver. Other organs such as the spleen, heart and brain are affected rarely [4,5]. Bone and soft-tissue hydatid disease are rare form of presentation of hydatid disease and account for around 1 - 5% of all cases. The environment in muscles is toxic to the establishment and growth of the cestode because of the increased concentration of the lactic acid and the movement of the muscles. Most of the muscular hydatid disease are secondary to visceral involvement and primary muscular hydatid diseases is even rarer, with most common sites being the neck, trunk and roots of the limbs [6,7]. We report a rare case of a primary intramuscular hydatid cyst of femoral muscles in a man who presented as a mass in the right thigh, making diagnosis very difficult.
Staging/Classification
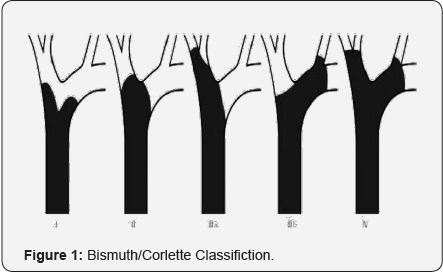
Over years, there were a number of staging/classification system, including Bismuth/Corlette, Liver Cancer Study Group of Japan, MSKCC (Memorial Sloan Kettering Cancer Center), AJCC (American Joint Committee of Cancer), and most recently European HPBA (European Hepato-Pancreato-Biliary Association). The most well-known classification was from Dr. Henry Bisthmus (Figure 1) [4], who classified the tumor according to the anatomic locations:
- Type 1 - tumor involves hepatic bile duct only;
- Type 2 - tumor involves bile duct bifurcation;
- Type 3a - tumor involves bile duct burfication and right hepatic bile duct;
- Type 3b - tumor involves bile duct burfication and left hepatic bile duct;
- Type 4 - tumor involves both sides of hepatic bile ducts. This system is used widely in clinical practice. It was based on anatomic level or involvment of the tumor to biliary tree. This does help in preparation of surgical plan. However, it does not describe the involvement status of vessels, status of liver parenchyma, metastasis and lymphnode. Therefore, it is not as helpful when liver resection and vascular resection/reconstruction are considered.
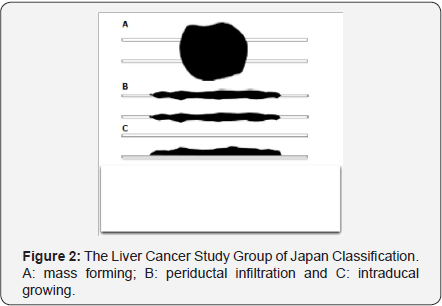
In 2000, the Liver Cancer Study Group of Japan proposed a classification according cancer growing pattern, mass forming, periductal infitration and intraductal growing (Figure 2) [5]. This was more about cancer biological behaviour, with better prognosis of mass forming and intraductal growing. Preoperatively, the information about this classification would unlikely be obtained in detail. Further more, there was no description about vascular involvement and liver lobar status. Therefore, the value of this classification in surgical assessment regarding resectability was limited.
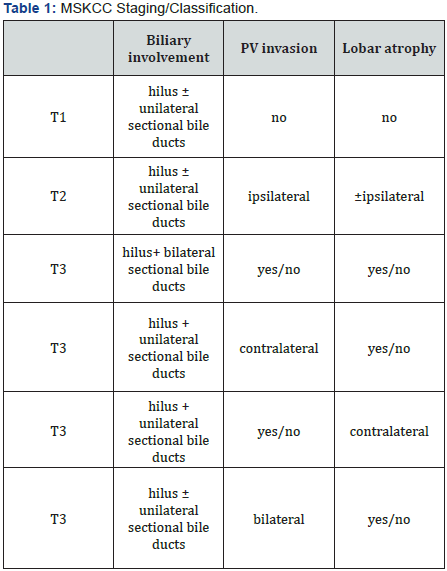
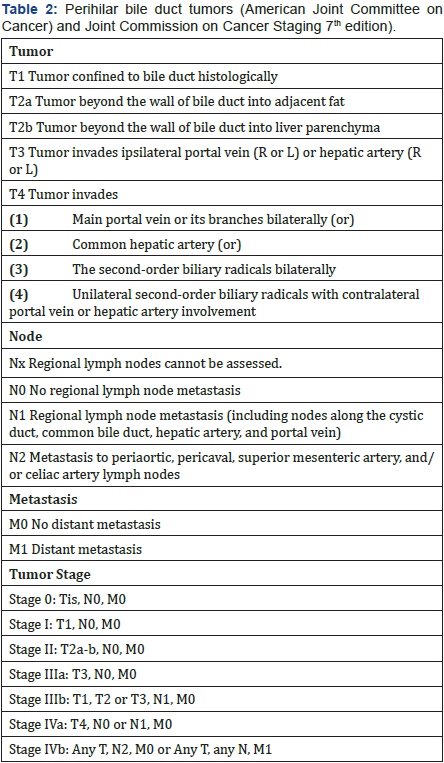
MSKCC staging/classification took bile duct involvement, vascular involvement and liver atrophy into consideration (Table 1) [6]. It helps in preparation of liver resection in treatment of Klatskin tumor. However, this classification did not include lymphnode and metastasis status, and did not consider liver transplantation as option of treatment. The AJCC’s TNM classification based on tumor, lymphnodes involvemen and distance metastasis [7] (Table 2). TNM is often used for most of the cancer staging. However, it is mostly appropriate for postoperative pathological staging, as some information such as tumor invasion, lymphnode metastasis, may not be accurately assessed before surgery.
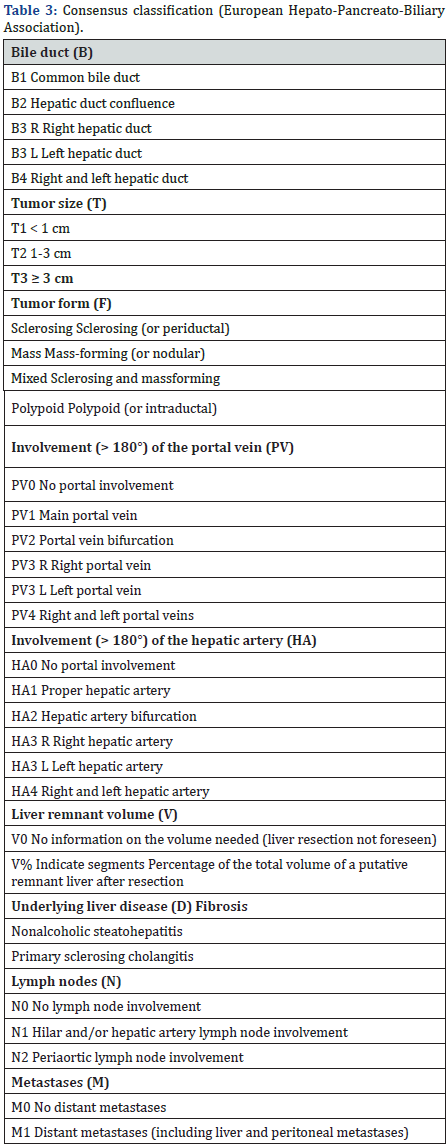
In 2011, an international working group based on a consensus from the European Hepato-Pancreato-Biliary Assoiation, published a new most complete staging/classification system [8] (Table 3) [16]. This is not only including information about bile duct, hepatic artery and portal vein, but also covering tumor biological growing form, liver resection related information such as liver volume, remnant volume and, background liver disease history. This system seems very complicated with some information not easily and accurately obtainable before resection.
Surgical management update
Original local resection of hilar cholangiocarcinoma (Klatskin tumor) had poor long term results with 5 year survival <7.3% [9]. In 1990’s, more and more studies showed R0 resection resulted longer survival. Klatskin tumor is found to have relatively low distance metastasis and high local recurrences. Therefore, combining radical choledochectomy with liver lobectomy was promoted, especially including right hemi- or right extended hemihepatectomy plus caudate lobe resection [10,11]. The 5 year survival rate increased to over 30-40%. However, postoperative morbidity and mortality were as high as 59% and 11%. In 2009, Chen XP et al. [12] however, published a paper on British Journal of Surgery, suggesting minor limited hepatectomy for patients whose cancers were not involving vascular structures might not be necessarily worse in outcome, with 5-yr survival rate of 34%, while the postoperative morbidity and mortality from a major extended resection were greatly avoided [12].
Issue of vascular resection and reconstruction has been reviewed through a mega-analysis by Abass S and Sandrassi C [13]. There were 669 patients out of 2457 cases had vascular resection, 22-88% resected sample were found positive on pathology, 36-88% of patients achieved R0 resection while the morbidity and mortality were respectively 22-88% and 2-15%. Five year survival was 20-56%. In 2012, Jong MC et al. [14] reported a multi-institutional analysis of 305 cases [14], showing portal vein resection should be undertaken when necessary to extirpate all disease. Combined liver resection, extra-hepatice bile duct resection and portal vein resection can offer long-term survival in some patients with advanced hilar cholangiocarcinoma.
Liver transplantation for treatment of cholangiocarcinoma has been attempted for more than twenty years. Before 2000, however, five year survival was only 28%. With neo-adjuvant therapy, the results were gradually improving. In 2005, Rea SR et al. [15] from Mayo Clinic with tedious strict preoperative selecting and treatment protocol [15]. The results was significantly better, with 1, 3, 5 year survival as 92%, 82% and 82%. Liver transplantation as a treatment option though in a small highlyselected group of patient, achieves R0 resection more readily without concerning about lobar atrophy, intrahepatic bile duct or vascular involvment.
Proposal of New Clinical Classification
Former staging/classifications did not take fully consideration of therapeutic process and are limited on description of cancer status related surgical management. Any final results of a cancer patient treatment is actually determined by three parts [15]:
- patient’s systemic healthy with range of tolerance of therapeutic actions;
- the cancer itself and its response to therapies; and
- the therapies.
This new clinical classification is based on clinical therapeutic options according to patient’s condition and cancer status. As therapeutic technologies including neoadjuvant therapy, chemoradiation and transplantation, are continuously improving, with further advances in therapeutic technologies in the future, more vpatients will receive radical treatment with better final results. In order to take all present available therapeutic options into consideration, a new clinical therapeutic classification is here proposed (Table 4).
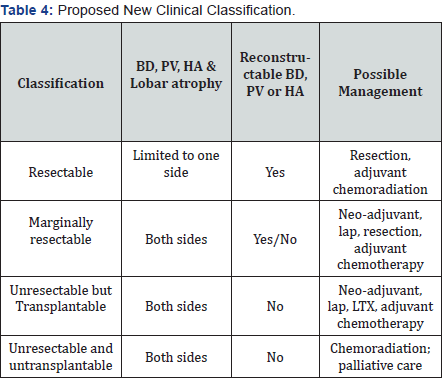
BD: Bile Duct; PV: Portal Vein; HA: Hepatic Artery; lap: Exploratory Laparotomy; LXT: Liver Transplantation
This relatively simple classification is to help in making clinical decisions regarding therapeutic purpose. The patients with Klatskin tumor are classified in 4 groups: resectable, marginally resectable, unresectable but transplantable and, unresectable and untransplantatable. Surgical treatment of hilar cholangiocarcinoma is not only depending on resection, but also on reconstruction. We could do as much resection as possible, as long as vascular and biliary reconstruction is achievable. Liver transplantation is useful when reconstruction becomes not possible. However, in order to achieve long-term good outcome with liver transplantation, liver transplantation could only be used in highly-selected patient group without distance metastasis, at present i.e. biologically less aggressive with 3-months neoadjuvant therapy with no progress and pathologically localized tumor with exploratory laparotomy -finding of no positive lymphnode.
Conclusion
Advances in management of hilar cholangiocarcinoma (Klatskin Tumor) leads to a new clinical classification, which will facilitate in clinical therapeutic decision at present time.

Comments
Post a Comment